Superfine powders refers to materials with micron to nanometer particle sizes. In 광물 processing, ultrafine powder means 100% 입자 크기 less than 30μm. Nanomaterials show unique properties like size effect and macroscopic quantum tunneling. These properties make nanomaterials widely used across many fields. However, nanomaterials have large specific surface areas and high activity. They are very unstable and easily agglomerate, losing their original properties. Agglomeration reduces material value and limits performance. It also increases difficulty in preparation and storage of nanomaterials. Therefore, agglomeration is a key technical challenge in nanomaterials development.

Agglomeration of superfine powders
Agglomeration of superfine powders means primary particles connecting into larger clusters. This occurs during preparation, separation, handling, and storage processes. Currently, three main causes of ultrafine powder agglomeration are recognized. First, intermolecular forces cause ultrafine powder agglomeration. Second, electrostatic forces between particles lead to agglomeration. Third, particles bond together when suspended in air.
Intermolecular forces cause superfine powders agglomeration
When mineral materials are ultrafine, particle distances become extremely short. Van der Waals forces then far exceed the particle’s own gravity. Thus, ultrafine particles tend to attract and agglomerate. Hydrogen bonds and adsorbed moisture bridges on particle surfaces also cause adhesion. Other 화학적인 bonding effects further promote particle aggregation.
Electrostatic forces between particles cause agglomeration
During ultrafine processing, mineral materials gain charges from impact and friction. Newly formed ultrafine particles accumulate large amounts of positive or negative charges. Some surface protrusions carry positive charges, others carry negative charges. These charged particles are highly unstable. To stabilize, they attract each other and contact at sharp points. This connection causes particle agglomeration. Electrostatic force is the main driving force in this process.
Particle bonding in air
When relative humidity exceeds 65%, water vapor condenses on particle surfaces. Liquid bridges form between particles, greatly enhancing agglomeration.
Additionally, during grinding, mineral materials absorb large amounts of mechanical or thermal energy. Thus, new ultrafine particles have very high surface energy. Particles are highly unstable in this state. To reduce surface energy, particles tend to aggregate and move closer. This also easily causes particle agglomeration.
Nanomaterial agglomeration is divided into soft agglomeration and hard agglomeration. Soft agglomeration is caused by intermolecular forces and van der Waals forces. It is relatively easy to eliminate soft agglomeration. There are five theories explaining hard agglomeration formation. They include capillary adsorption, hydrogen bonding, and crystal bridge theories. Also, chemical bonding and surface atom diffusion bonding theories exist. However, no unified explanation has been established yet. Currently, many studies focus on dispersion technologies to prevent superfine powders agglomeration.
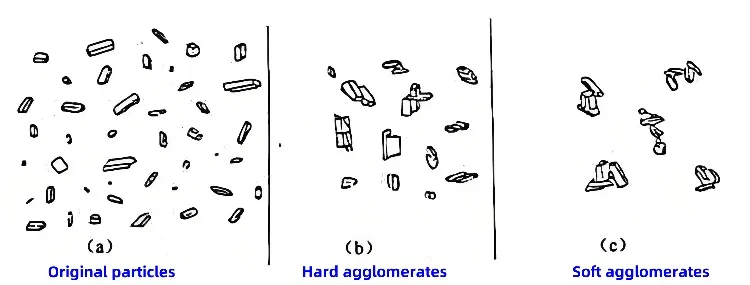
Dispersion of superfine powders
Superfine powders dispersion mainly concerns two types of dispersion states.
One is dispersion in a gas medium. The other is dispersion in a liquid medium.
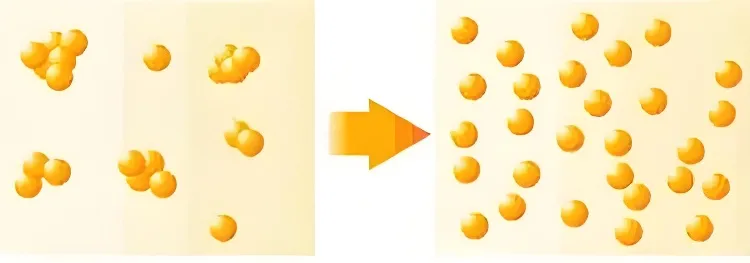
Dispersion method in liquid phase
Mechanical dispersion method
Mechanical dispersion uses external shear or impact forces to disperse nanoparticles in a medium. Methods include grinding, 볼밀, 핀밀, 에어젯밀, and mechanical stirring.
The main issue with mechanical stirring is that particles may reaggregate when leaving the turbulence. Once the particles exit the turbulent field, the external environment may cause them to reform clusters. Therefore, using mechanical stirring with chemical dispersants often yields better dispersion results.

Chemical dispersion method
Chemical dispersion is a widely used method for dispersing ultrafine powder suspensions in industrial production. By adding inorganic electrolytes, surfactants, and polymer dispersants, the powder surface is modified.
This alters the interaction between the powder and the liquid medium, achieving dispersion.
Dispersants include surfactants, small-molecule inorganic electrolytes, polymer dispersants, and coupling agents. Among them, polymer dispersants are the most commonly used, with polyelectrolytes being the most important.
Ultrasonic method
Ultrasonic control places the industrial suspension in an ultrasonic field. By adjusting the frequency and duration, particles are fully dispersed. Ultrasound is more effective in dispersing nanoparticles. Ultrasonic dispersion uses cavitation to generate high temperature, pressure, shockwaves, and microjets. These weaken the interaction forces between nanoparticles, preventing agglomeration and ensuring dispersion. However, excessive ultrasonic stirring should be avoided. With increased heat and mechanical energy, particle collisions increase, causing further agglomeration.
Dispersion methods in the gas phase
Drying and dispersion
In humid air, liquid bridges between powder particles are the main cause of agglomeration. Drying solid materials involves two basic processes. First, heat is applied to the material to vaporize the moisture. Second, the vaporized water diffuses into the gas phase. Therefore, preventing liquid bridge formation or breaking existing bridges is key to ensuring dispersion. Most powder production processes use heating and drying as a pretreatment step.
Mechanical dispersion
Mechanical dispersion refers to using mechanical force to break apart agglomerated particles. The necessary condition is that the mechanical force (shear and compressive stress) must exceed the adhesion force. Typically, mechanical force is generated by high-speed rotating impeller disks or high-speed air jet impact . This results in strong turbulent airflow motion. Such as 에어젯밀 그리고 핀밀 etc.
Mechanical dispersion is relatively easy to achieve. However, it is a forced dispersion method. While agglomerated particles can be broken apart in the disperser, their interactions remain unchanged. After leaving the disperser, particles may reaggregate. Additionally, mechanical dispersion may crush brittle particles. As mechanical equipment wears down, dispersion efficiency decreases.
Electrostatic Dispersion
For homogeneous particles, surface charge similarity causes electrostatic repulsion. Thus, electrostatic forces can be used for particle dispersion. The key issue is how to fully charge the particle group. Methods like contact charging and induction charging can charge particles. The most effective method is corona charging. This method uses corona discharge to form an ion curtain, charging particles. The particles receive the same polarity charge. Electrostatic repulsion between charged particles disperses them.
결론
There are many other methods for ultrafine powder modification, differing greatly from the mainstream methods. However, regardless of the method, further research on modification principles is required. The goal is to find new methods suitable for various modification needs and practical production.
This requires optimizing modification processes based on in-depth understanding of modification mechanisms. We need to develop “composite” treatment processes that can achieve multiple modification goals. Moreover, modifications to existing general chemical equipment are needed to adapt to surface modification. In conclusion, this requires cooperation and continuous progress across the entire powder industry, academia, and research.
에픽파우더
에픽 파우더, 초미립자 분말 산업에서 20년 이상의 업무 경험. 초미립자 분말의 미래 개발을 적극적으로 촉진하고, 초미립자 분말의 파쇄, 분쇄, 분류 및 수정 공정에 중점을 둡니다. 무료 상담 및 맞춤형 솔루션을 원하시면 저희에게 연락하세요! 저희 전문가 팀은 고품질 제품과 서비스를 제공하여 분말 처리의 가치를 극대화하는 데 전념합니다. Epic Powder—신뢰할 수 있는 분말 처리 전문가!
