In 1989, SONY discovered petroleum coke could replace lithium in rechargeable batteries. This marked the start of large-scale lithium-ion battery applications. Research on anode materials began from that point. Over the next 30 years, three generations of anode materials emerged. These include carbon, lithium titanate, and silicon-based materials. This article classifies Lithium Battery Anode Materials by structure and briefly introduces their characteristics and performance. It also reviews progress in material improvements and development directions. The focus is on next-generation high-energy-density anode materials. Future trends and current status of these materials are highlighted.

Carbon Materials
Carbon materials are the most widely used commercial Lithium Battery Anode Materials today. They mainly include natural graphite, artificial graphite, hard carbon, soft carbon, and MCMB. Before next-generation anodes mature, carbon—especially graphite—will remain the mainstream choice.
Graphite
Graphite is divided into natural and artificial types based on raw materials and processing methods. Due to low lithium potential, high initial efficiency, good cycling stability, and low cost, graphite has become the ideal anode material for current lithium-ion battery applications.
Natural graphite: Typically uses natural flake graphite as raw material, processed into spherical graphite through modification.
Although widely used, natural graphite has several drawbacks: Many surface defects and large specific surface area lead to low initial efficiency. With PC-based electrolytes, severe co-intercalation of solvated lithium ions occurs, causing layer expansion and exfoliation. Strong anisotropy limits lithium insertion to edge planes, resulting in poor rate performance and lithium plating risk.
Modification of natural graphite:
To address surface defects and poor electrolyte tolerance of natural graphite, various surfactants are used for modification.
To address strong anisotropy in natural graphite, industrial production often uses mechanical shaping for spheroidization. Jet mill uses air impact to cause particle collisions and trim sharp edges. This method avoids impurity doping and offers high spheroidization efficiency.
However, it causes significant particle pulverization, leading to low yield.

Artificial graphite: Typically made from dense petroleum coke or needle coke precursors, avoiding surface defects found in natural graphite. However, it still suffers from poor rate performance, low-temperature behavior, and lithium plating due to crystal anisotropy. Unlike natural graphite, artificial graphite is modified by restructuring particle morphology to reduce the orientation index (OI). Commonly, 8–10 μm needle coke is used as a precursor, with pitch or similar graphitizable binders. Through rotary kiln treatment, several particles are bonded into secondary particles (D50: 14–18 μm), then graphitized, effectively lowering the OI value.
Soft carbon
Soft carbon, also known as graphitizable carbon, refers to amorphous carbon materials that can be graphitized above 2500 °C. Depending on the precursor’s sintering temperature, soft carbon can form three crystal structures: amorphous, turbostratic (disordered), and graphite structure—the latter being typical artificial graphite. Amorphous soft carbon, with low crystallinity and large interlayer spacing, has good electrolyte compatibility. As a result, it offers excellent low-temperature performance and good rate capability, drawing widespread attention.
Soft carbon has a high irreversible capacity during the first charge and discharge, a lower output voltage, and no distinct charge/discharge plateaus. As a result, it is generally not used independently as an anode material but rather as a coating or component.
Hard carbon
Hard carbon, also known as non-graphitizable carbon, is difficult to graphitize even at temperatures above 2500°C. It is typically produced by heat treatment of precursors at 500–1200°C. Common types of hard carbon include resin carbon, organic polymer pyrolysis carbon, carbon black, and biomass carbon. Phenolic resin, when pyrolyzed at 800°C, forms hard carbon with an initial charge capacity up to 800 mAh/g, and a d002 interlayer spacing greater than 0.37 nm (compared to 0.3354 nm for graphite). The larger interlayer spacing facilitates lithium-ion insertion and extraction, giving hard carbon excellent charge/discharge performance. This makes hard carbon a new research focus for anode materials. However, its drawbacks include high initial irreversible capacity, voltage plateau hysteresis, low tap density, and the tendency to generate gas, which cannot be overlooked.
Lithium titanate material
Lithium titanate (LTO): Lithium titanate (LTO) is a composite oxide composed of metallic lithium and the low-potential transition metal titanium. It belongs to the AB₂X₄ series of spinel-type solid solutions. LTO has a theoretical specific capacity of 175 mAh/g, with an actual specific capacity greater than 160 mAh/g. It is one of the Lithium Battery Anode Materials that have already been commercialized.
Advantage
Zero strain property: LTO has a lattice parameter a = 0.836 nm. During charge/discharge, lithium insertion/extraction has minimal impact on its crystal structure. This prevents structural changes from volume expansion/contraction, giving it excellent electrochemical stability and cycle life.
No lithium plating risk: LTO has a high lithium insertion potential of 1.55 V. No SEI film forms during initial charge, resulting in high first-cycle efficiency, good thermal stability, low interface resistance, and excellent low-temperature performance—can charge at -40°C.
3D fast ion conductor: LTO has a 3D spinel structure, with lithium pathways much larger than graphite’s interlayer spacing.
Its ionic conductivity is an order of magnitude higher than graphite, making it ideal for high-rate charge/discharge.
Disadvantage
LTO also has drawbacks due to its low specific capacity and voltage plateau, resulting in low energy density. Its nanostructured form is highly hygroscopic, causing severe gas generation and poor high-temperature cycling. The material fabrication process is complex and costly. As a result, LTO cell costs are more than three times higher than those of equivalent-energy LFP (lithium iron phosphate) cells.
Application of materials
LTO’s advantages and disadvantages are both very pronounced, with performance characteristics that are quite extreme. Therefore, it is best applied in specific niche fields where its strengths can be fully utilized. Currently, LTO batteries are mainly used in urban pure electric BRT buses, electric hybrid buses, and power grid frequency regulation and peak-shaving services.
Silica-based material
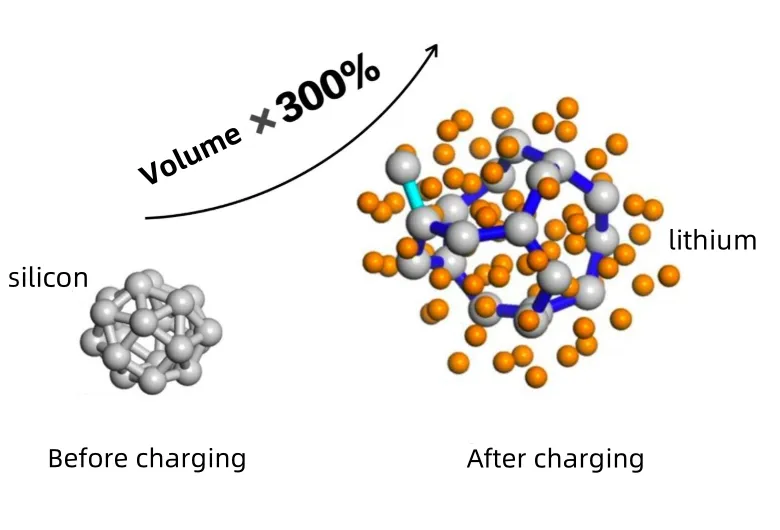
Silicon is considered one of the most promising Lithium Battery Anode Materials, with a theoretical specific capacity of up to 4200 mAh/g—over 10 times that of graphite. Its lithium insertion potential is higher than that of carbon, reducing the risk of lithium plating and improving safety. Current research focuses on two main directions: nano-silicon carbon composites and silicon oxide (SiOx) anode materials.
Application challenges:
- Huge volume expansion and contraction during lithiation/delithiation cause particle pulverization and electrode structure damage, leading to electrochemical performance failure.
- Continuous SEI film breaking and reforming due to volume changes consume electrolyte and reversible lithium, accelerating capacity fade and drastically lowering charge/discharge efficiency.
To solve these issues, researchers have been actively exploring new methods to improve silicon anode performance. The mainstream approach is to use graphite as the base material and add 5%–10% by mass of nano-silicon or SiOx. These are then coated with carbon to suppress volume changes and enhance cycling stability.
Conclusion
This paper summarizes the structural characteristics and functional features of various lithium-ion battery anode materials. It reviews recent research progress on different anode materials used in lithium-ion batteries. With continuous improvement and modification, silicon-based materials have emerged as the most promising next-generation anodes. However, their inherent large volume expansion and poor cycle performance hinder large-scale application.
Many recent modification methods face challenges such as complex processes and high costs. This calls for deeper understanding of fundamental principles and the development of simple, efficient methods to produce composite nano-silicon materials. The goal is to create lithium-ion batteries with low expansion, high initial efficiency, high rate capability, and safety—paving the way for silicon anodes to replace graphite and achieve breakthroughs in electric vehicle applications.
Epic powder
Epic Powder, 20+ years of work experience in the ultrafine powder industry. Actively promote the future development of ultra-fine powder, focusing on crushing,grinding,classifying and modification process of ultra-fine powder. Contact us for a free consultation and customized solutions! Our expert team is dedicated to providing high-quality products and services to maximize the value of your powder processing. Epic Powder—Your Trusted Powder Processing Expert !
